Cement requires water to undergo hydration. However, after hydration is complete, excess water can dissolve the hydration products, causing leaching and weakening the structure. This issue is particularly critical for water-retaining concrete structures. For example, in dams, hydrostatic pressure and water permeability can cause the rapid leaching of these hydration products. This article examines the leaching of hydration products, focusing on water-retaining structures.
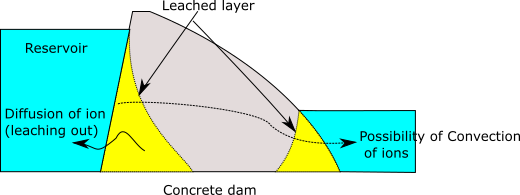
Water can diffuse into the concrete and dissolve both hydration products and aggregates. The dissolved materials will then diffuse out due to concentration gradients. Soft water (with low dissolved minerals) or water containing aggressive chemicals, such as acids or chlorides, has a greater leaching effect.
Types of leaching
Halvorsen (1966) performed studies on lime leaching in water retaining concrete
structures. Halvorsen divides leaching into three types:
1) Leaching through very porous concrete,
2) Leaching from free concrete surfaces,
3) Leaching through cracks in concrete.
What can be leached out?
The leaching susceptibility of sodium (Na), potassium (K), and calcium (Ca) varies with the ionization capacity of water. Since calcium is a primary component of hydration products like alite and belite, significant leaching can reduce the strength of concrete.
Moskvin (1980) showed that leaching can continue if the leached water evaporates, as seepage typically seals off unless re-exposed by external water, such as rain. This phenomenon occurs due to the deposition of salts in cracks and their subsequent carbonation.
Factors affecting leaching
- Water permeability
- The hardness of the water
- Total Ca in the concrete and the amount of Ca(OH)2
- Any additives that can bind lime
- Carbonation of Ca(OH)2
- The amount of carbonic acid which is free to attack the concrete.
Leaching process
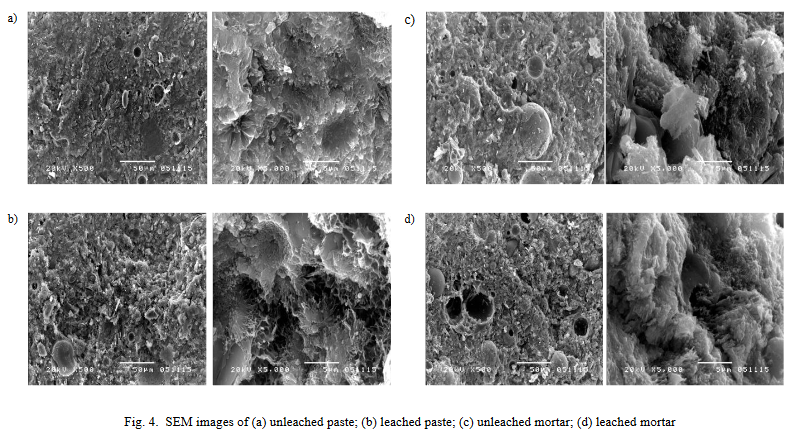 The SEM image from Worachai Ponloa (2018) illustrates both leached and unleached cement paste and mortar.
The SEM image from Worachai Ponloa (2018) illustrates both leached and unleached cement paste and mortar.
Moskvin (1980) studied the concentration of leached lime from concrete pores, and the results are presented in the figure below. At slow flow rates, the flow becomes saturated with calcium (Ca). However, as the flow rate increases, the leaching of Ca stabilizes because water must diffuse into the smaller pores to extract calcium. This indicates that the diffusion process becomes dominant at higher flow rates.
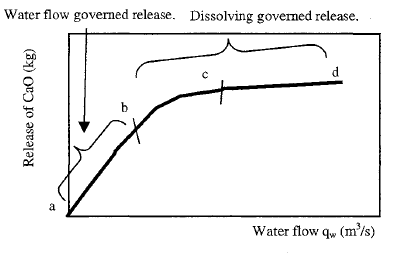
The solubility of ions from the cement matrix is a thermodynamic process influenced by temperature and pressure. Higher water temperatures facilitate the rapid leaching of calcium (Ca) ions compared to cooler water. These dissolved ions can exit the matrix through convection (due to water flow through permeable pores) or diffusion toward the surface driven by concentration gradients. Clearly, leaching rates are higher during convection due to the increased availability of water.
Carbon dioxide (CO2) can easily dissolve in water to form carbonic acid (H2CO3), which lowers the pH. This increased acidity enhances the solubility of alkaline compounds like calcium hydroxide (Ca(OH)2) and other calcium products in the CSH matrix.
As discussed in another article, leaching of calcium from the matrix can significantly weaken concrete—affecting both compressive and tensile strength—and poses a serious risk of reinforcement corrosion. This leaching phenomenon can be detected if observed carefully, particularly during dry periods when leached products (efflorescence) are visible on the surface.
Core testing is a direct method to assess the extent of leaching and carbonation in concrete, which typically occurs concurrently.
A case study
Swedish Concrete Institute has published technical report (in 2001) on leaching performance of concrete. One of them is described below.
In a concrete core extracted from an 85-year-old water tank at a filtration plant (constructed in 1906), it was observed that approximately 10 mm of the surface had been damaged due to leaching. An interesting finding was the redistribution of calcium (Ca), with flow from the inner concrete toward the Ca-deficient zone, leading to the formation of secondary ettringite. It’s important to note that in earlier construction, the cement content was typically higher than it is today, which likely allowed for a greater amount of Ca to be leached out. However, most of the leaching appears to have occurred through the diffusion process, as indicated by the relatively shallow depth of leaching (only about 10 mm).









