Pottery vessels have been crafted for about 18,000 years, but what transforms raw clay into a colourful pot? And what chemistry lies behind the pottery?
Pottery is one of humanity’s oldest crafts. The earliest known fired clay object, a small figurine, dates back almost 30,000 years, while the oldest pottery vessel was made around 18,000 years ago. Over millennia, pottery evolved across the globe for both practical uses and artistic expression. Around 7,000 years ago, the Egyptians pioneered the art of glazing, followed by significant improvements in kiln technology by the Chinese, leading to the production of highly decorated stoneware and porcelain.
These advancements were achieved through centuries of trial and error, and although modern science has helped us understand the composition and structure of the materials, pottery-making remains heavily influenced by empirical techniques due to the complexity of its processes.
Shaping the Pot
The earliest pottery was made by hand, but the potter’s wheel, invented around 7,000 years ago, became a widely used tool. Clay, composed largely of water, can be shaped because its molecular structure allows layers to slip past one another when force is applied. This plasticity is due to the water molecules that separate layers of clay sheets, held together by hydrogen bonds.
As the clay dries, water escapes, and the layers bond more tightly, forming a stronger structure known as “greenware.” However, until fired, the clay remains vulnerable to water and can be reworked if soaked again.
Clay Composition
Clay originates from feldspar, a group of minerals that make up about 60% of the Earth’s crust. Over time, feldspar weathers through water action, producing clay minerals. The primary clay mineral in pottery is kaolinite, (K2O.Al2O3.6SiO2) composed of silicon and aluminium oxides arranged in a sheet-like structure. The structure of kaolinite and its transformations during the firing process are key to understanding pottery’s physical changes.
Firing Process
The process of firing clay creates cross-links between hydroxyl groups, turning it into a solid, permanent form. Oxides of first-row transition metals like iron, cobalt, and copper are the primary sources of colour in pottery glazes, creating a wide palette of shades.
The firing process is transformative. Initially, at around 500°C, chemically bound water in the clay is driven off, causing irreversible changes. If firing stops here, the clay can no longer be reshaped but remains fragile. Higher firing temperatures (around 1,000°C) produce “biscuit ware,” a strong but porous material, which can then be glazed and fired again.
<clay>-OH + HO-<clay> → <clay>-O-<clay> + H2O(gass)
The final firing determines the type of pottery: earthenware (1,000-1,150°C) or stoneware and porcelain (>1,200°C). At higher temperatures, the clay undergoes further chemical changes, including the formation of mullite crystals and the vitrification of feldspar, which strengthens the pot.
Glazes
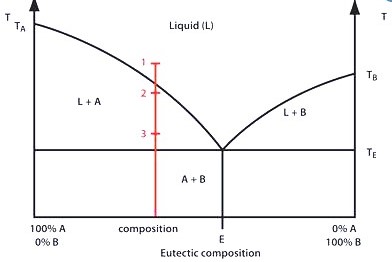
Glazes, typically made from silicon dioxide, aluminium oxide, and fluxes, form a glassy coating that enhances both aesthetics and functionality. Potters carefully control texture, opacity, and colour, often using transition metal oxides for colouration. The phase diagram of a glaze helps potters predict how much of the glaze will melt and solidify during firing, affecting the final texture and appearance.
Colour Chemistry
Pure clay minerals are colorless, so the vibrant hues in pottery glazes come from oxides of transition metals. For example, iron oxide can produce red, yellow, brown, blue, or green tones depending on firing conditions. Copper oxide, another common colouring agent, appears blue or green in an oxidizing atmosphere, but under reducing conditions, it shifts to reddish tones due to the formation of elemental copper.
The chemistry of pottery is an intricate balance of materials, temperatures, and atmospheric conditions, offering endless possibilities for artistic and scientific exploration.
References:
- A Paul, Chemistry of glasses, 2nd edn, p336-342, London, Chapman & Hall, 1990
- S A T Redfern, Clay Miner., 1987, 22, 447 http://bit.ly/M3cstt (pdf)
- Stephen Breuer. (2012) The chemistry of pottery | Feature | RSC Education. Retrieved September 28, 2024, from https://edu.rsc.org/feature/the-chemistry-of-pottery/2020245.article








