The phenomenon of aggregates reacting with the alkali component of cement is called alkali aggregate reaction (AAR). The predominant form of alkali aggregate reaction is alkali-silica reaction (ASR) in which the silica content of aggregate reacts with sodium and potassium hydroxide of concrete. Other forms of AAR are alkali-carbonate reaction and alkali-silicate reaction. In China and Canada, alkali-carbonate reaction has been a greater cause for concern; in this reaction, the argillaceous dolomitic limestone aggregate containing calcite and clay reacts with alkali. Alkali-silicate reaction is significantly less prevalent.
ASR reaction occurs in a limited extent in many concrete structures, but damage due to ASR is rare. The reaction exhausts itself, leaving the structure in deteriorated but serviceable condition. Nonetheless, any cracks that are formed due to this reaction enhances the rate of other form of deterioration, such as corrosion and carbonation.
History and geographical aspects
ASR was first presented by Poulsen in January 1914 to the Institution of Danish Civil Engineers. The phenomenon was further studied in the 1930s in the USA and presented by Stanton in 1940 by giving examples of structures in California. Stanton also demonstrated by tests about the expansive nature of this reaction. The ASR phenomenon was gradually discovered in other countries such as Denmark (1950s), Germany (1960s), UK (1970s), France (1970s), South Africa (1976), Norway (1980s), etc.
The aggregate of many countries appeared to be innocuous, however, when time favoured, the reaction occurred and the problem became apparent. It must be mentioned that the problem started to appear when the cement content was gradually increased to achieved higher concrete strength (higher cement means higher alkali percentage). With the understanding of the reaction, guidelines and codes has been progressively introduced and updated in most of the countries since 1980s.
Detecting ASR
The ASR reaction occurs in almost all concrete structure in varying degree, but does not always cause damage. In other words, damage due to ASR is a more rare occurrence than ASR itself. In fact, some damages that were attributed to ASR were subsequently diagnosed as delayed ettringite formation or other forms.
ASR cracking is described as pattern or map cracking and is usually accompanied by a dark strain adjacent to the crack on the surface. The document published by US Department of Transportation could be useful to identify ASR in the field; this document can be downloaded from this link.
The ASR reaction occurs in almost all concrete structure in varying degree, but does not always cause damage
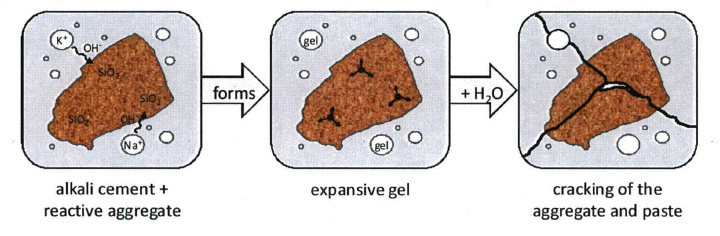
ASR Chemistry
Silicon di oxide (SiO2) is present in most of the aggregates in various forms such as quartz, tridymite or cristobalite. The reactivity of these minerals varies. One interesting feature of silica is that a unique structural relationship exists between SiO2 and H2O which enables water to substitute to some extent for silica, thus forming an amorphous hydrous silica which has higher affinity to alkali. In the presence of moisture, the oxygen atom on the surface of the aggregate are hydroxylated and replaced by loose silanol bonds
Si-O-Si + H2O –> Si-OH . . . OH-Si
The alkalies in cement are in the form of Sodium Oxide (Na2O) and potassium oxide (K2O) in about 0.2-1.3 % in OPC. This alkali dissolve in the pore liquid during hydration forming sodium, potassium and hydroxyl ions. Furthermore, the anhydrous calcium oxide of the cement, CaO, reacts with water to produce C-S-H and calcium hydro-oxide to produce Ca(OH)2. Ca(OH)2 may also dissolve in the pore liquid ad Ca2+ and 2OH– ions. However, the high alkalinity of the solution due to Na+OH– and K+OH– renders Calcium very insoluble. The pH of such solution can be as high as 12.4 at 20C. Note that the higher alkanity of the solution is due to presence of OH- ions.
These OH- ions reacts with loose silanol bonds to give further hydrooxyls:
Si-OH+OH – —> SiO– + H2O
The negative SiO- further attracts Na+, K+ and Ca2+ cations to balance the charge, which further loosens the silica lattice. The penetration of hydroxyl and sodium ion cause a breakdown of Si-O-Si bonds, liberating silica and allowing a way for penetration of larger molecules of calcium.
An insoluble gel is formed as Na+ joins the negative oxygen ion of the silica surface. If the environment is rich in Ca+ content, the gel absorbs Ca2+ and gets transformed into a rigid and unreactive structure. If the pore solution is low in Ca2+, the gel attracts more Na+ and K+ resulting in a more viscous gel, this gel is capable of further attracting water and causing large expansion. If the gel is in contact with CO2, it carbonates, which often happen at the surface, giving the distinct black color near the crack.
The factors affecting ASR reaction rate are
a) an adequate supply of moisture
b) availability of silica
c) sufficient alkali in pore
Moisture Content
The moisture content in the gel pore firstly acts as a transport route for reactive ions. And secondly, the moisture is absorbed by the gel causing expansion. Generally, relative humidity greater than 80% helps the ASR phenomenon.
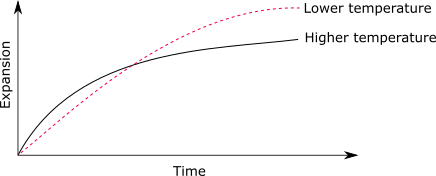
Temperature effect
An elevated temperature increases the rate of reaction in the initial phase, but gradually declines with time. At lower temperature, the rate of reaction is low, but the total expansion has been observed to be higher. The reaction tends to mature at about 20 years.
Aggregate Porosity
It has been found that the low porosity aggregate will more damaging effect due to its inability to accommodate expansion.
Tests
- Aggregate assessment and petrographic examination is essential
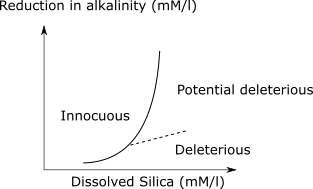
- b) Chemical test using ASTM C289 (Standard Test Method for Potential Alkali-Silica Reactivity of Aggregates (Chemical Method))- the test involves grinding the aggregate and treating with NaOH at 80C for 24 hours. Measurement are made of the dissolved silica and reduction in alkalinity of the sodium hydroxide solution. The data is plotted and location is determined in a standard curve.
Other tests are
- ASTM C1260: “Test Method for Potential Reactivity of Aggregates (Mortar-Bar-Test)”. It is a rapid test of aggregates: immersion of mortar bars in NaOH 1 M at 80 °C for 14 days used to quickly identify highly reactive aggregates or quasi non-reactive aggregates.
- ASTM C1293: “Test Method for Concrete Aggregates by Determination of Length Change of Concrete Due to Alkali-Silica Reaction”. It is a long-term confirmation test (1 or 2 years) at 38 °C in a water-saturated moist atmosphere (inside a thermostated oven) with concrete prisms containing the aggregates to be characterised mixed with a high-alkali cement specially selected to induce ASR. The concrete prisms are not directly immersed in an alkaline solution, but wrapped with moist tissues and tightly packed inside a water-tight plastic foils.
- ASTM C1567: “Standard Test Method for Determining the Potential Alkali-Silica Reactivity of Combinations of Cementitious Materials and Aggregate (Accelerated Mortar-Bar Method)”
Pessimum effect
There is a high expansion of concrete when the silica content reaches certain value, below or above which the expansion is lowered. This phenomenon is called pessimum effect. When the alkali content is low, the volume of gel produced is low, thus expansion is low. When the alkali content is high, the reaction is vigorous and exhaust itself before the concrete hardens, thus accommodating all the expansion.
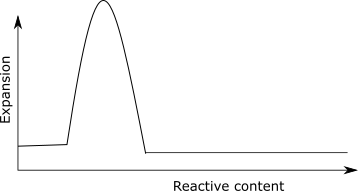
It has been found that the maximum volume expansion of silica gel in sodium hydroxide solution occurs at an intermediate level of total SiO2/Na2O mole ratio.
There is a high expansion of concrete when the silica content reaches certain value, below or above which the expansion is lowered.
Impact in structural behaviour
ASR induced cracking will slightly reduce the strength of material in compression, tension and flexure by about 10-30%. This is more apparent in the case of reinforced structures. For reinforced structures, which are confined by reinforcement or any other methods, the effect of ASR has not been found significant.
Prevention
ACI 201.2R-0l recommends the use of a low alkali cement (not more than 0.6% alkali) and the use of a suitable pozzolanic material, as prescribed by ASTM C 618-05. However, it must be noted that the resulting concrete should not exhibit an increase in shrinkage or a reduced resistance to frost damage (with air entrainment).







